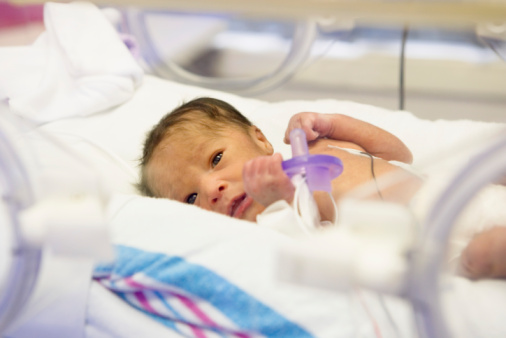
Back in April, I wrote a blog post for The Doctor’s Tablet about a controversy regarding the ethics of informed consent for research in a study of treatments for extremely premature newborns. The study compared different oxygen levels given to preemies in an attempt to determine the optimal level for this high-risk group.
At the time, I had access only to an article about the study that appeared in the New York Times, as well as the letters from the Office for Human Research Protections (OHRP) to the investigators in the study. I argued then that the OHRP was justified in criticizing the informed-consent forms for research carried out in more than 20 neonatal intensive care units in prestigious university medical centers in the United States.
To my surprise, the controversy has grown, with no fewer than four articles published in the New England Journal of Medicine (NEJM) defending both the study and the informed-consent documents.
This is an example of a situation in which, as we in bioethics frequently say, “reasonable people can disagree.”
The first two NEJM articles were a Perspective piece by two well-known bioethicists, Arthur Caplan and David Magnus, and an editorial (subscription required) by Dr. Jeffrey Drazen, the editor in chief of NEJM. Neither article analyzed the consent documents, nor did they provide detailed information about the studies themselves. Two weeks later, two more articles appeared. One was a long letter signed by 46 bioethicists and pediatricians, the other an article by three top officials at the National Institutes of Health (NIH), including Francis Collins, the director. Like the first two articles, these two defended the importance of studying extremely premature newborns, lamented the possible damage the OHRP’s action could do to future research and defended the wording in the consent forms because the procedures in the research were all “standard of care” for these patients.
Reflecting on the list of the 46 signatories to the bioethicists’ letter published in the NEJM, I saw the names of many colleagues and friends. Their bottom line was that the OHRP had “overreached” in the determination letter sent to the researchers who conducted the study, and that the consent forms for the study were mostly adequate “but could use improvement.”
After these articles were published in the NEJM, two colleagues and I took the other side of the issue. We examined the OHRP’s letter to the researchers, the NIH-sponsored research protocol for the study and all of the consent forms used by the medical centers where the research was conducted. We found those consent forms to be uniformly flawed. They failed to disclose foreseeable risks of the study about which the parents of their premature infants should have been told before deciding to enter their babies in the study. We did not criticize the ethics of the study itself. Informed consent remains a bedrock requirement in determining the ethics of research with human beings. Our criticisms were published recently in an NEJM Correspondence, of which I am the corresponding author.
The letter from the 46 bioethicists and pediatricians also stated that the OHRP had no business questioning the adequacy of consent forms approved by internal review boards (IRBs), the committees responsible for the review of research in U.S. medical centers that conduct research. That critique assumes that IRBs are infallible in their determination that consent forms adequately comply with the U.S. code of federal regulations for research.
What, then, is the role of the OHRP? The agency, located within the U.S. Department of Health and Human Services, describes on its website its role: providing “leadership in the protection of the rights, welfare, and well-being of subjects involved in research.” So if the ORHP finds deficiencies in research sponsored by the U.S. government, the appropriate response is to act on its findings.
I think that’s a perfectly worthy mission, and one that is well worth a respectful disagreement with colleagues and friends.

