According to the American Diabetes Association, approximately 1.25 million Americans have type 1 diabetes (T1D). Five percent of people with diabetes have this form of the disease; it is usually first diagnosed in children and young adults, affecting their health and hampering their lifestyle. For those of us caring for these patients and trying to find new ways to help improve glycemic control, recent approval by the Food and Drug Administration (FDA) of a new technology is bolstering our research and treatment efforts.
How “closed loop” systems work
In T1D, the pancreas does not produce insulin. That lack of insulin leads to the accumulation of glucose in the bloodstream instead of allowing it to flow to the cells, where it can be used for energy. Patients with T1D are currently treated using multiple daily subcutaneous insulin injections (MDI) or insulin pump therapy, which provides continuous subcutaneous insulin infusion (CSII). Without basal, or background, and bolus insulin administration, people with T1D would not survive.
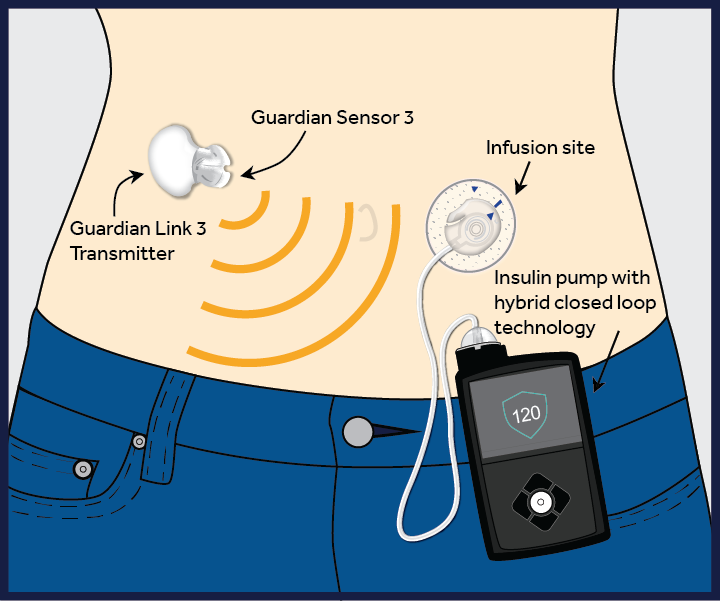
Medtronic Hybrid CL system diagram
An important innovation, the closed loop (CL) or “artificial pancreas” system, consists of an insulin pump (a medical device that infuses insulin subcutaneously through a catheter reinserted every two to three days) and a CGM, or continuous glucose monitor (which checks the patient’s glucose in the interstitial fluid every minute). The CGM communicates with the insulin pump and, using an algorithm, prompts the insulin pump to infuse insulin based on the glucose value, how fast the glucose level is changing and the amount of insulin already given.
The hybrid CL system, which connects the pump and the monitor directly, reduces the burden on the patient of making calculations and decisions. Currently, patients without CGM (CSII or MDI) base their insulin doses on the real-time values of their blood glucose. This does not account for any rise or fall in the glucose level. Patients also must consider exercise, stress and any additional life circumstances that can influence glucose levels. This is difficult to do, and patients are not always able to determine the correct doses. Patients with CGM currently are able to use the information provided on the device, such as a rise or fall in glucose level, to help them make their decisions. However, these decisions remain a burden to patients living with this chronic illness 24 hours a day for their entire lives. The new hybrid CL system could help relieve some of that burden.
This is why the FDA’s recent announcement approving the first hybrid CL system for T1D, from Medtronic, is exciting, as patients and doctors can now believe that a full CL system is within reach. The hybrid CL system’s unique technology will permit improved glucose control with decreased user input around the clock, based on the individual’s personal basal insulin. It will suggest increases or decreases in basal insulin based on the glucose level, the rate of change in the glucose level and the amount of insulin still active in people with T1DM, significantly assisting patients with their decision making. Patients must still enter mealtime carbohydrate counts to receive a meal bolus (the specific amount of insulin needed at meals), and they must occasionally calibrate the sensor using a blood glucose value or finger stick.
Providers can tailor the technology to help keep patients safe. Glucose control is affected by many things, from stress to illness, so even in the most well-controlled patients, having glucose in a healthy range all the time is impossible. This new technology may help make this goal a reality.
An encouraging future
At Montefiore, the division of pediatric endocrinology and diabetes has been involved in research on the use of technology in caring for patients with diabetes over the past six years. Though not yet perfect, our own CL efforts in patients with T1D have shown remarkable results. Seeing a CL system in action—even in the developmental stage—suggests how life-changing this can be for patients and healthcare providers. The success of the hybrid system reinforces our goal of creating a fully automated CL system.
Hybrid CL systems and taking the next step
Twenty years ago, CL was not even a consideration, so the hybrid CL device represents a huge step forward for our patients living with and having to think about T1D 24 hours every day.
There is definitely significant promise here for T1D patients and their physicians. However, as with all technology and newly approved health treatments, further refinements and adjustments will be needed. Insurance coverage will be an issue for the majority of our patients, as CGM is not currently covered. Risk of human error or technology malfunction will still have to be kept in mind daily, as certain errors can cause life-threatening diabetes ketoacidosis. None of these risks, however, minimizes the importance of the FDA’s approval of a hybrid CL system and the hope it brings to all patients living with type 1 diabetes.

