Editors’ Note: Susan Band Horwitz, Ph.D., was named a recipient of the 2019 Canada Gairdner International Awards for her pioneering research into Taxol, establishing the mechanism of action of the blockbuster chemotherapy drug, which has been used to treat cancers of the ovary, breast, and lung. Read the related news release and watch the video about Dr. Horwitz’s research. In connection with this honor, Dr. Horwitz reflected on her experiences related to her research into Taxol. This post first appeared in Cell.com.
 When I was finishing up my graduate studies at Brandeis University, my plan was to pursue the field of enzyme kinetics after I received my PhD in biochemistry. But after giving birth to twins 5 days after defending my thesis, I decided my plan required a short-term adjustment. My thesis advisor, professor Nathan O. Kaplan, helped me to find a position that would accommodate a flexible schedule while my boys were young. As a result, I began conducting research in the Department of Pharmacology at Tufts University Medical School, with the expectation that I would move back into my intended field in a few years. But I was hooked. This was my serendipitous entry into the world of pharmacology and natural-products research.
When I was finishing up my graduate studies at Brandeis University, my plan was to pursue the field of enzyme kinetics after I received my PhD in biochemistry. But after giving birth to twins 5 days after defending my thesis, I decided my plan required a short-term adjustment. My thesis advisor, professor Nathan O. Kaplan, helped me to find a position that would accommodate a flexible schedule while my boys were young. As a result, I began conducting research in the Department of Pharmacology at Tufts University Medical School, with the expectation that I would move back into my intended field in a few years. But I was hooked. This was my serendipitous entry into the world of pharmacology and natural-products research.
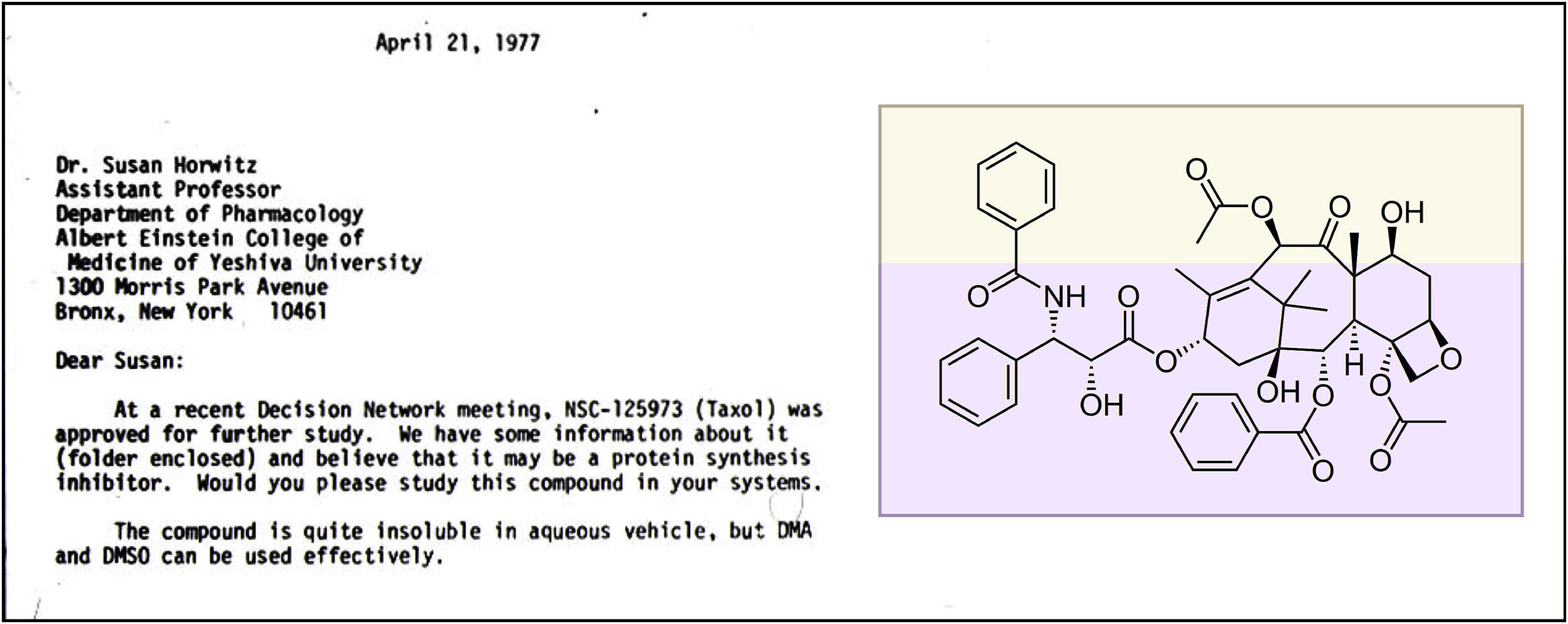
Several years later, in 1977, when I was an assistant professor at Albert Einstein College of Medicine, I received a letter from the National Cancer Institute (NCI) requesting that I look at a molecule named Taxol.
My group had a track record of examining the mechanisms of action of natural products with anticancer properties: we had previously published on camptothecin, etoposide, and bleomycin. Although I had never heard of Taxol, the structure was intriguing: architecturally complex and extraordinarily hydrophobic. I like to say it is a structure that only a tree could make. The molecule had been isolated from the bark of the Pacific yew tree (Taxus brevifolia) by two superb natural-product chemists, Drs. Monroe Wall and Mansukh Wani, who worked at the Research Triangle Institute in North Carolina. They were able to purify the compound and determine its correct structure, which was quite a feat in the 1960s, particularly since the molecule was present in very low quantities in tree bark. Their 1971 landmark paper in the Journal of the American Chemical Society was the single paper on Taxol in the literature when my laboratory began to study the compound. (Today there are over 34,000 papers with the word Taxol or its generic name, paclitaxel, in the title and/or abstract.)

Dr. Horwitz with Peter Schiff, 1980
At the time, a new graduate student in my laboratory, Peter Schiff, was looking for a thesis project. I suggested he look into Taxol and requested 10 mg from the NCI. I also told him that if after 1 month of experiments the compound did not look interesting, we should look for a new project. Needless to say, that day has not arrived.
We started with some simple experiments. First, how good was the drug at killing cells? At 10 −9 M Taxol, a very low concentration of drug, the proliferation of HeLa cells was inhibited after 24 h due to mitotic arrest. I suggested to Peter that we look at the treated HeLa cells by electron micro- scopy. At that time, there was only one electron microscope in the school, in our Department of Anatomy. Half an hour after Peter left, I received an urgent call from him: ‘‘Come upstairs to the Department of Anatomy immediately!’’
The cells treated with Taxol were chock full of microtubule bundles. It was a ‘‘hallelujah’’ moment for me and one of the most exciting days of my career, since we had identified a new and dramatic phenotype. For Peter, it became obvious that he had a thesis project. Such stable microtubule bundles are indicative of Taxol treatment and are seen in the white blood cells of patients who are receiving the drug.
Further investigation indicated that Taxol has a binding site on the microtubule polymer and that when Taxol is bound to that site, microtubules are stabilized and incapable of undergoing normal cycles of polymerization and depolymerization. It became evident to me that if we were to understand how Taxol stabilized microtubules, we would have to characterize its binding site. The question was how to accomplish this task, since Taxol did not make a covalent bond with microtubules. I knew a challenging path lay ahead.
Around that time, I was invited to speak at a meeting in Hawaii that was devoted to understanding the mechanism of action of bleomycin, an antitumor drug particularly useful in treating testicular cancer in combination with etoposide and cisplatin. My group, in a collaboration with Jack Peisach’s laboratory at Albert Einstein, had been studying this drug extensively and had demonstrated a role for ferrous iron and oxygen in the degradation of DNA by bleomycin.
At the meeting, I was introduced to John Douros, head of the drug development branch at the NCI. I told him how excited I was to be working with Taxol and that I believed it just might have potential as a new antitumor therapy. I was particularly enthusiastic because it had a mechanism of action not previously observed in an antitumor drug. I also told him that my work would be much easier if I had Taxol with a radiolabeled tag, to better follow the interaction of the drug with microtubules. Dr. Douros suggested that I write him a letter with my request and he would try to obtain radiolabeled Taxol for me. I sent a letter as soon as I returned to Einstein, but I never received a reply. In the meantime, I wrote to every pharmaceutical company in the United States, not requesting money but rather asking whether they might have a chemist willing to work with me to label Taxol. Not a single company replied.
A dozen years later, when Dr. Wall was cleaning out his office in anticipation of his retirement, he sent me a note asking whether I had ever seen Dr. Douros’s letter to him. The casual disrespect in that letter remains disturbing to me even now. Even at that early stage of my career, I clearly was not a ‘‘poor girl.’’
But in the laboratory, research with Taxol continued. A new graduate student, Jerry Parness, joined my lab and was able to add a tritium label at the 7-position on Taxol, a site that we knew from structure-activity studies would not alter the anticancer activity of the drug. Our experiments indicated that the binding site for Taxol was on the β-tubulin subunit of microtubules. This set the stage for the next phase of our research done in collaboration with the late George Orr at Albert Einstein, in which three radiolabeled photoaffinity derivatives of Taxol were used to help deepen our understanding of Taxol’s binding site in β-tubulin.
In retrospect, it is truly amazing that Taxol became an important cancer drug, considering all the strikes against it. When my group published a series of papers in the early 1980s delving into the interaction of the compound with microtubules, it was the cell and molecular biologists who were anxious to get their hands on the compound. (They realized it would be an excellent tool to study the cellular cytoskeleton, of which microtubules are an essential component). There was essentially no interest from the clinical community.
But the NCI selected Taxol for clinical development, based primarily on activity against a mouse model of B16 melanoma, and approved it for a new drug application in 1982. When clinical trials sponsored by the NCI started, several problems arose. First of all, there was a severe shortage of the drug because the tree produces very small quantities and purification was complex. But the most serious problem was that many patients had severe side effects, including anaphylactic reactions. As a result, clinical studies were suspended for 5 years.
During this hiatus, oncologists, medicinal chemists, and pharmacologists worked together to move the drug forward. It was determined that patients should be pretreated with antihistamines and steroids coupled with delivery over a 24 h period instead of as a short infusion, as was done originally. Once that protocol was established and clinical trials resumed, it became clear that Taxol had significant antitumor efficacy in patients with cancer.
Many drugs that presented only some of the problems associated with Taxol have never made it to the clinic. I believe that, because Taxol had a mechanism of action not previously described, everyone involved was encouraged to push this drug forward despite the obstacles.
But after the successful trials, a very unusual situation emerged, one that many young scientists today might find difficult to understand. No one held a patent on Taxol, and therefore, no pharmaceutical company was interested in developing the drug. To overcome this problem, the NCI developed a Cooperative Research and Development Agreement (CRADA) with Bristol-Myers Squibb, which competed for and received the right to commercially develop Taxol. To its credit, the company increased the supply of the drug by introducing a semisynthetic synthesis that was first developed by Robert Holton at Florida State University and also expanded clinical trials worldwide. In 1992, the Food and Drug Administration (FDA) approved Taxol for refractory ovarian cancer, 21 years after the original 1971 paper by Drs. Wall and Wani and their collaborators. In 1994, the FDA approved Taxol for breast cancer and, in 1997, for AIDS-related Kaposi’s sarcoma and non-small cell lung cancer in combination with cisplatin.
Today, my goal is to understand why some patients respond to Taxol and others do not. We know there are eight β-tubulin human isotypes expressed differentially in cells and tumors, so we are investigating whether Taxol interacts with these isotypes in unique ways. We already know that Taxol binds less effectively to one β-tubulin isotype compared to the others, so it is possible that the response of a tumor to the drug could depend partially on its isotype content. However, I am well aware from our studies over many years that drug resistance is extremely complex and has many components.
It has been more than 4 decades since I received the initial letter from the NCI, and I have thoroughly enjoyed studying Taxol and contributing knowledge about a drug that has improved and saved the lives of many cancer patients around the world. I acknowledge with great pleasure the students and fellows who have joined me in my laboratory. It has been my privilege to work with them. I particularly want to acknowledge Chia-Ping Yang and Hayley McDaid, with whom I have collaborated over many years.